*Home Delivery and Retail Pharmacy
$70 per month pricing applies to 90-day prescriptions only. Please note that 90-day prescriptions are not available in all states. $89 home delivery pharmacy pricing includes 6-week New Patient Packs, 6-week Titration Packs and all 30-day prescriptions. Additional shipping and handling costs will apply. VIVUS LLC reserves the right to modify or discontinue these offers at any time without notice. The QsymiaEngage.com home delivery pharmacy is a cash only program - insurance claims will not be processed. Please see QsymiaEngage.com for full program details. LifeLine Specialty Pharmacy is NOT AVAILABLE to patients in Puerto Rico, Guam or the U.S. Virgin Islands. GoodRx is a wholly owned trademark of GoodRx, Inc. and is neither sponsored by nor affiliated with the makers of Qsymia. Costco pricing and program is exclusive to Costco Members paying cash.
**IQVIA Reporting - Jan 2023 - Dec 2025
Qsymia is a prescription medicine that contains phentermine and topiramate extended-release. Qsymia may help adults and children 12 years and older with obesity, or some adults with overweight who also have weight-related medical problems, to help them lose excess body weight and keep the weight off.
- Qsymia should be used with a reduced calorie diet and increased physical activity.
- It is not known if Qsymia changes your risk of heart problems or stroke or of death due to heart problems or stroke.
- It is not known if Qsymia is safe and effective when taken with other prescription and over-the-counter medicines, or
herbal weight loss products.
- It is not known if Qsymia is safe and effective in children under 12 years old.
Do not take Qsymia if you are pregnant, planning to become pregnant, or become pregnant during Qsymia treatment; have glaucoma; have thyroid problems (hyperthyroidism); are taking certain medicines called monoamine oxidase inhibitors (MAOIs) or have taken MAOIs in the past 14 days; are allergic to topiramate, sympathomimetic amines such as phentermine, or any of the ingredients in Qsymia. See the end of the Medication Guide for a complete list of ingredients in Qsymia.
Birth defects (cleft lip/cleft palate). If you take Qsymia during pregnancy, your baby has a higher risk for birth defects called cleft lip and cleft palate. These defects can begin early in pregnancy, even before you know you are pregnant. Patients who are pregnant must not take Qsymia. Patients who can become pregnant should have a pregnancy test before taking Qsymia and every month while taking Qsymia and use effective birth control (contraception) consistently while taking Qsymia. Talk to your healthcare provider about how to prevent pregnancy.
If you become pregnant while taking Qsymia, stop taking Qsymia immediately, and tell your healthcare provider right away. Healthcare providers and patients should report all cases of pregnancy to FDA MedWatch at 1-800-FDA-1088, and the Qsymia Pregnancy Surveillance Program at 1-888-998-4887.
Qsymia may slow the increase in height in children 12 years and older.
Suicidal thoughts or actions. Topiramate, an ingredient in Qsymia, may cause you to have suicidal thoughts or actions. Call your healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you: thoughts about suicide or dying; attempts to commit suicide; new or worse depression; new or worse anxiety; feeling agitated or restless; panic attacks; trouble sleeping (insomnia); new or worse irritability; acting aggressive, being angry, or violent; acting on dangerous impulses; an extreme increase in activity or talking (mania); other unusual changes in behavior or mood.
Serious eye problems, which include any sudden decrease in vision, with or without eye pain and redness or a blockage of fluid in the eye causing increased pressure in the eye (secondary angle closure glaucoma). These problems can lead to permanent vision loss if not treated. Tell your healthcare provider right away if you have any new eye symptoms.
Visual field defects (independent of elevated intraocular pressure) have been reported in clinical trials and in postmarketing experience in patients receiving topiramate. In clinical trials, most of these events were reversible after topiramate discontinuation. If visual problems occur at any time during treatment, consider discontinuing Qsymia.
Qsymia may cause a severe rash with blisters and peeling skin, especially around the mouth, nose, eyes, and genitals (Stevens-Johnson Syndrome). Qsymia may also cause a rash with blisters and peeling skin over much of the body that may be life threatening (Toxic Epidermal Necrolysis). Call your healthcare provider right away if you develop a skin rash or blisters.
Numbness or tingling in the hands, arms, feet, or face (paraesthesia); dizziness; changes in the way foods taste or loss of taste (dysgeusia); trouble sleeping (insomnia); constipation; and dry mouth.
Depression, dizziness, joint pain, fever, flu, and ankle sprain.
Mood changes and trouble sleeping. Qsymia may cause depression or mood problems, and trouble sleeping. Tell your healthcare provider if symptoms occur.
Concentration, memory, and speech difficulties. Qsymia may affect how you think and cause confusion, problems with concentration, attention, memory or speech. Tell your healthcare provider if symptoms occur.
Increases of acid in bloodstream (metabolic acidosis). If left untreated, metabolic acidosis can cause brittle or soft bones (osteoporosis, osteomalacia, osteopenia), kidney stones, can slow the rate of growth in children, and may possibly harm your baby if you are pregnant. Metabolic acidosis can happen with or without symptoms. Sometimes people with metabolic acidosis will: feel tired, not feel hungry (loss of appetite), feel changes in heartbeat, or have trouble thinking clearly. Your healthcare provider should do a blood test to measure the level of acid in your blood before and during your treatment with Qsymia.
Central Nervous System (CNS) side effects. The use of prescription sleep aids, anxiety medicines, or drinking alcohol with Qsymia may cause an increase in CNS symptoms such as dizziness and light-headedness. Do not drink alcohol with Qsymia.
Possible seizures if you stop taking Qsymia too fast. Seizures may happen in people who may or may not have had seizures in the past if you stop Qsymia too fast. Your healthcare provider will tell you how to stop taking Qsymia slowly.
Kidney stones. Drink plenty of fluids when taking Qsymia to help decrease your chances of getting kidney stones. If you get severe side or back pain, and/or blood in your urine, call your healthcare provider.
Decreased sweating and increased body temperature (fever). People should be watched for signs of decreased sweating and fever, especially in hot temperatures. Some people may need to be hospitalized for this condition.
Qsymia capsules contain the inactive ingredient FD&C Yellow No. 5 (tartrazine) which can cause allergic-type reactions (including bronchial asthma) in certain people, especially people who also have an allergy to aspirin.
Tell your healthcare provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Qsymia. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to VIVUS LLC at 1-888-998-4887 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please read the
Qsymia Medication Guide,
Full Prescribing Information and
Risk of Birth Defects with Qsymia Patient Brochure.
1. Qsymia Full Prescribing Information. Campbell, CA: VIVUS LLC; 2025.
2. Data on File. VIVUS LLC.
3. Kumar S, Kelly AS. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin Proc. 2017 Feb;92(2):251-265.
4. Cardel MI, Jastreboff AM, Kelly AS. Treatment of Adolescent Obesity in 2020. JAMA. 2019 Nov 5;322(17):1707-1708.
5. Centers for Disease Control (n.d.). Prevalence of Childhood Obesity in the United States. Retrieved from https://www.cdc.gov/obesity/data/childhood.html
6. Rush EC, Yan MR. Evolution not Revolution: Nutrition and Obesity. Nutrients. 2017 May 20;9(5):519.
7. Amin T, Mercer JG. Hunger and Satiety Mechanisms and Their Potential Exploitation in the Regulation of Food Intake. Curr Obes Rep. 2016 Mar;5(1):106-12.
8. Halford JCG, Bereket A, Bin-Abbas B, Chen W, Fernández-Aranda F, Garibay Nieto N, López Siguero JP, Maffeis C, Mooney V, Osorto CK, Reynoso R, Rhie YJ, Toro-Ramos M, Baur LA. Misalignment among adolescents living with obesity, caregivers, and healthcare professionals: ACTION Teens global survey study. Pediatr Obes. 2022 Jul 15:e12957.
9. Puhl RM, Himmelstein MS. Adolescent preferences for weight terminology used by health care providers. Pediatr Obes. 2018 Sep;13(9):533-540.
10. Pont SJ, Puhl R, Cook SR, Slusser W; SECTION ON OBESITY; OBESITY SOCIETY. Stigma Experienced by Children and Adolescents With Obesity. Pediatrics. 2017 Dec;140(6):e20173034. American Academy of Pediatrics.
11. Lessard LM, Puhl RM. Weight-based cybervictimization: Implications for adolescent health. Pediatr Obes. 2022 Jun;17(6):e12888. The study involved 452 U.S. adolescents aged 11–17 years old with an average age 14.91 years.
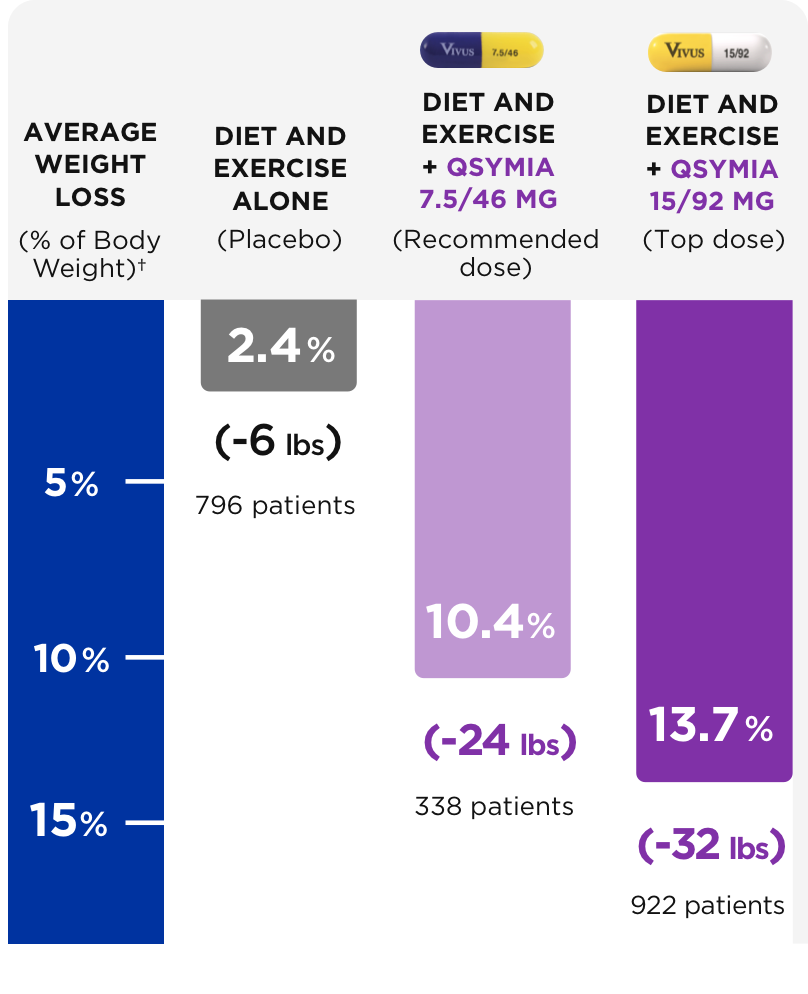
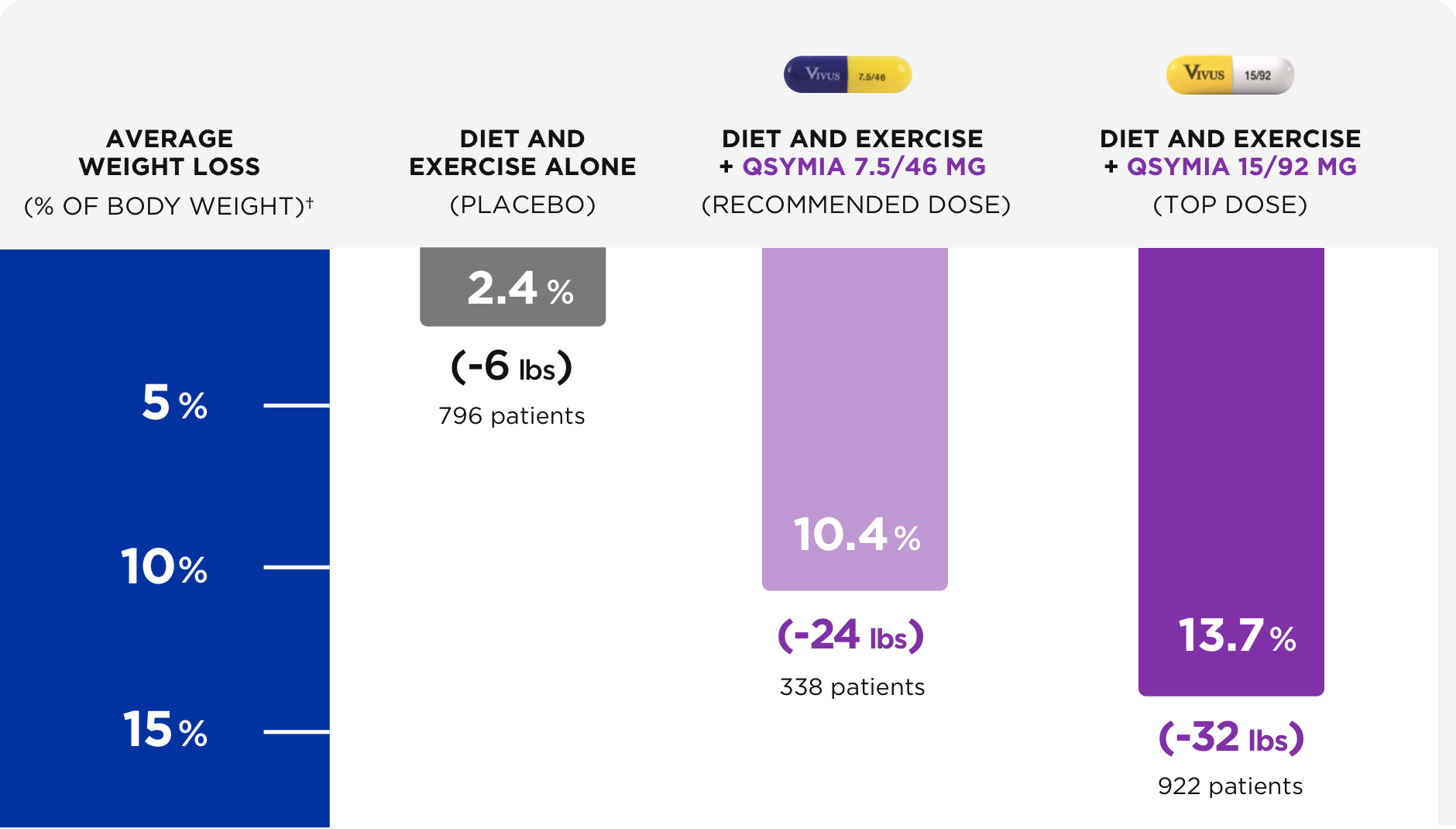
12. Gadde KM, Allison DB, Ryan DH, Peterson CA,Troupin B, Schwiers ML, Day WW. Lancet. 2011 Apr 16;377(9774):1341-52.


![ASK ABOUT A NON INJECTION[SM] WEIGHT-MANAGEMENT TREATMENT OPTION](/patient/include/image/global/adult_non_injection@2x.png)
